Amendment to the regulation
on prior notification for export of medicines
Was amended the law on prior notification for export of medicines.
This amendment seeks to improve the mechanism of prior notification, in order to clarify some concepts and criteria for inclusion and exclusion of medicinal products covered by this Regulation.
The main changes to the regulation are:
- a statement that the objective criteria on which is based the inclusion of drugs covered;
- reduction of administrative character of different entities (in particular in the reporting of transactions by marketing authorisation holders and distributors of drugs);
- definition of a frequency for updating the list of drugs subject to prior notification, in order to make more evident the monitoring that the Infarmed makes of the mechanism.
The list of drugs has also changed, with the output of 10 medicinal products (corresponding to 16 registration numbers of Marketing Authorization, because there are several presentations of the same medicine) and entry of 2 drugs (corresponding to 2 registration numbers AIM).

Infarmed discloses information
about use of vancomycin.
Recommendations on the use of Vancomycin-containing medicinal products issued by the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) have been posted on the Infarmed website. These recommendations are based on a review of the indications of use and approved dosages and are disseminated in the context of combating antimicrobial resistance and following the completion of the review of the safety and efficacy information for all vancomycin-containing medicinal products referred to users.
In Portugal, vancomycin-containing medicinal products are for hospital use only and are administered by injection, infusion and oral route.
You can access the disclosed information here.

Opening of Public Tender for
Installation of a mobile pharmaceutical
In order to widen the network of places authorized for the commercialization of medicines, a public tender was launched in Diário da República for the installation of a mobile pharmaceutical unit in the town of Muge, in the municipality of Salvaterra de Magos , District of Santarém.

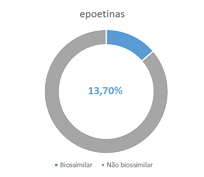
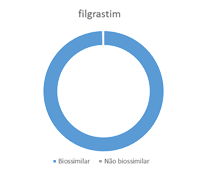
Increases consumption of biosimilars
The market for biosimilars is growing.
Of note is infliximab, which in 2016 reached a 25% share, an increase of 11 percentage points compared to 2015.
Biosimilar medications allow the treatment of more patients with the same financial resources.
.